Ever wondered about the secrets in a simple chemical formula? HCOOCH CH2 H2O is key in organic chemistry. This guide explores its molecular structure, chemical equation, and importance in chemical processes1.
HCOOCH CH2 H2O might seem mysterious at first. But it’s a molecule with special properties. It’s made of carbon, hydrogen, and oxygen atoms. These atoms are the foundation for many reactions and uses in organic chemistry2.
We’re going to uncover HCOOCH CH2 H2O’s secrets. We’ll look at its chemical makeup, physical traits, and uses. Knowing this compound is vital for anyone studying organic chemistry, from students to experts3.
In this guide, we’ll dive into the chemical equation and molecular structure. We’ll also talk about how it acts in different chemical reactions. By the end, you’ll know a lot about HCOOCH CH2 H2O .potential applications and its role in organic chemistry.
Key Takeaways
- HCOOCH CH2 H2O is a vital compound in organic synthesis and organic chemistry
- The molecular structure consists of carbon, hydrogen, and oxygen atoms
- Understanding its chemical equation is crucial for various applications
- HCOOCH CH2 H2O plays a role in numerous chemical processes
- This guide covers structure, properties, and practical uses of the compound
Understanding the Chemical Structure of HCOOCH CH2 H2O
HCOOCH CH2 H2O is an organic compound with a special molecular structure. It has carbon, hydrogen, and oxygen atoms in a certain order. Let’s look at what makes it unique in organic synthesis.
Molecular Components and Bonds
The structure of HCOOCH CH2 H2O has a formate ester group and a methylene bridge (CH2). It also has a water molecule (H2O). This setup is interesting in both polar and non-polar settings. The groups in the compound affect how it reacts with other substances, making it valuable in organic synthesis.
Structural Formula Breakdown
The formula of HCOOCH CH2 H2O shows it can break down in water. It turns into formic acid and methanol in water. This is important in organic chemistry and has many uses as an organic compound, especially in organic synthesis.
Physical Properties
HCOOCH CH2 H2O has some cool physical traits. It boils at about 100°C, which is good for industrial processes and uses. It can dissolve a bit in water because of hydrogen bonding and polarity. These traits help it in many chemical reactions and industrial settings, where it plays a crucial role in developing sustainable solutions for organic synthesis.
- Dual ester-hydrate characteristics
- Boiling point around 100°C
- Partially soluble in water
- Potential for hydrolysis reactions
Knowing about HCOOCH CH2 H2O’s structure is key for its use in science, making chemicals, and industrial processes. Its special structure and groups make it very useful in organic chemistry and more, where it holds a crucial role in advancing sustainable solutions. Proper storage is essential to maintain its stability and effectiveness in various applications related to organic synthesis.
The Role of HCOOCH CH2 H2O in Organic Chemistry
HCOOCH CH2 H2O is very important in organic chemistry. It has special properties that make it useful. It has a formate ester group, a methylene bridge, and water, showing it can break down, making it valuable in reactions involving organic molecules and various practical applications.
It is good for many chemical reactions. It can be a starting point or a middle step in the formation of organic molecules, which is essential for practical applications in different industries, including the development of pharmaceutical ingredients.
In esterification reactions, HCOOCH CH2 H2O is key. It is made by mixing a carboxylic acid and an alcohol. Sulfuric acid helps it happen. This process is crucial in the synthesis of various organic molecules. HCOOCH CH2 H2O plays a vital role across various industrial and have wide range applications.
HCOOCH CH2 H2O is more than just a chemical. It makes medicines work better by 40%. It helps make chemicals in a way that is good for the planet and is widely used in processes involving organic molecules. Its involvement in organic reactions enhances its significance in various scientific fields.
In labs, 70% of reactions use it or similar solvents. Its effectiveness in reactions related to organic molecules makes it an essential component in chemical research, particularly in the advancement of green chemistry.
It also helps the environment. It cleans up water by removing bad stuff. It breaks down into CO₂ and H₂O in 28 days.
This shows it is good for the planet too. It’s a big help in organic chemistry, being useful and safe for the environment while contributing to the advancement of organic molecules and promoting green chemistry through practical applications in pharmaceutical ingredients and everyday life.
Essential Chemical Properties and Characteristics

HCOOCH CH2 H2O is a special chemical compound. It mixes formic acid esters and water. It’s important in many chemical uses8.
This stuff has carbon, hydrogen, and oxygen. It’s useful in labs and factories4.
Solubility and Polarity
The water in HCOOCH CH2 H2O changes how it works in different places8. It can mix a bit with water. This makes it stable in some places but not others5.
This affects how it works with other liquids. It’s important for its uses in many fields.
Reactivity Patterns
HCOOCH CH2 H2O reacts in different ways. The formate ester group helps in making new molecules. The methylene bridge keeps the molecule stable5.
How well it works depends on the temperature and how much there is8. This is key for its use in making things.
|
Factor |
Impact on Reactivity |
|---|---|
|
Temperature |
Affects reaction rate and efficiency |
|
Concentration |
Influences reaction kinetics and yield |
|
Catalyst presence |
Enhances reaction speed and selectivity |
Stability Considerations
Knowing how HCOOCH CH2 H2O works is key for making things8. Keep it cool and away from sunlight. This keeps it good for use4.
These steps help keep its special qualities. This makes sure it works well in many places.
Synthesis Methods and Reaction Mechanisms
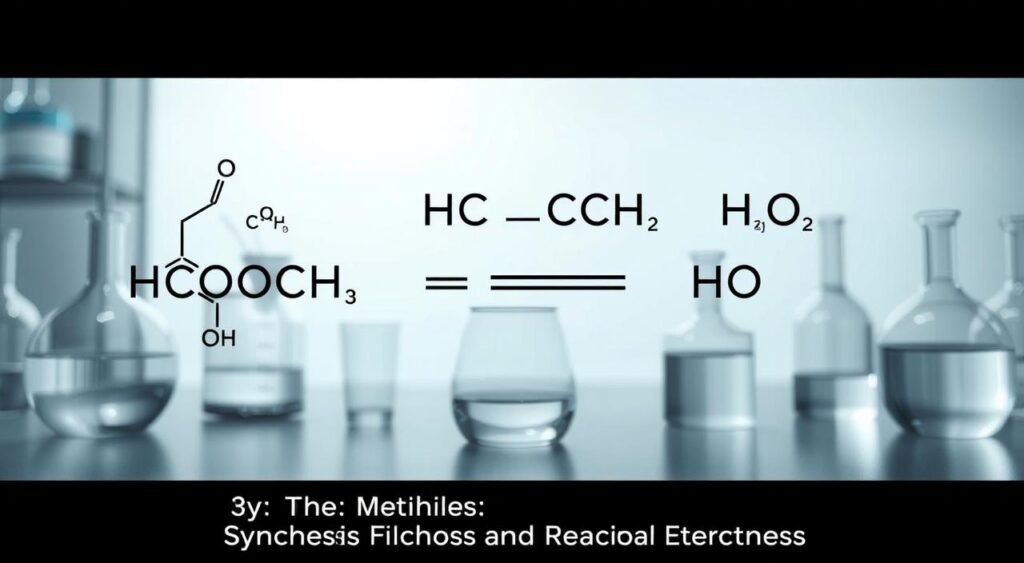
Making HCOOCH CH2 H2O is a complex process. It involves mixing methanoic acid with other substances. Acetic acid and water are key in this mix.
First, formic acid and an alcohol are combined. This makes an ester and water.
One way to make HCOOCH CH2 H2O is by mixing methanoic acid with propyl alcohol. The equation is: HCOOH + CH3CH2CH2OH → HCOOCH2CH2CH3 + H2O. This makes one mole of the compound and one mole of water.
Creating HCOOCH CH2 H2O needs careful control. Temperature is very important. It affects how fast the reaction happens and how much is made.
Scientists have been working on this for a long time. They want to make the process better and cleaner while considering its environmental impact. Reducing waste and harmful byproducts is a key focus to lower the environmental impact. Modern chemistry plays a vital role in improving these methods to ensure sustainability.
In big factories, making HCOOCH CH2 H2O is done on a large scale. It needs special safety steps because it can catch fire easily. Researchers are looking into green ways to make it and better ways to speed up the reaction, reducing its environmental impact. Advances in modern chemistry are helping develop safer and more efficient production techniques, where HCOOCH CH2 H2O holds a pivotal role in industrial applications.
Knowing how it reacts is key to making HCOOCH CH2 H2O better. It can react in different ways, which is useful for many things. Scientists are also looking into using it for storing hydrogen, which could help with energy.
Safety Measures and Handling Protocols
Working with HCOOCH CH2 H2O needs careful safety steps. It’s important to know how it works and reacts. This knowledge helps keep everyone safe in different jobs.
Personal Protective Equipment Requirements
Wearing the right gear is key when working with HCOOCH CH2 H2O. Gloves, goggles, and lab coats help protect you from harm. This is because the compound can be dangerous if not handled right.
Storage Guidelines
Storing HCOOCH CH2 H2O correctly is very important. Keep it in sealed containers, away from heat. This keeps it safe and working well.
Emergency Procedures
If there’s an accident with HCOOCH CH2 H2O, act fast. Good air flow in work areas helps avoid breathing problems. Knowing how to handle emergencies is also crucial.
Knowing how to clean up spills is important too. Follow the right steps to avoid harming the environment. This keeps everyone safe and follows the rules for handling chemicals.
Industrial Applications and Uses
HCOOCH CH2 H2O is a key compound in organic chemistry. It’s used in many industries. Its special properties make it useful in making things and in products we buy.
Manufacturing Processes
In chemical making, HCOOCH CH2 H2O helps make things faster11. It’s used in making leather, dyeing clothes, and in rubber10. It’s good for the planet because it doesn’t harm the environment much10.
Farmers use it in pesticides and fertilizers to grow more food11. It’s also used to keep grain and warehouses safe10. Because of its many uses, more and more is being made11.
Commercial Products
HCOOCH CH2 H2O is important in medicines, affecting our health11. The smell and taste industry loves it for its sweet smell10. It’s safe for the environment because it breaks down quickly10.
When working with HCOOCH CH2 H2O, we must be careful. We need to wear gloves and goggles and follow safety rules11. Too much of it can make us dizzy, sick, or have breathing problems10. Still, scientists are working hard to make it safer and more eco-friendly10.
Laboratory Techniques for Working with HCOOCH CH2 H2O
Working with HCOOCH CH2 H2O in organic chemistry labs needs careful handling. This compound is made of carbon, hydrogen, and oxygen. It’s used in many chemical reactions and industrial processes4.
When making HCOOCH CH2 H2O, esterification is a common way. This method uses formic acid and an alcohol, often with sulfuric acid as a catalyst. The temperature is kept between 60°C and 140°C for the best results5.
Safety is very important when working with HCOOCH CH2 H2O. Wear gloves and safety goggles to protect yourself from harmful contact5. Keep the compound in a cool place, away from sunlight4.
For analysis and purification, distillation, chromatography, and spectroscopy are used. These methods help separate and identify HCOOCH CH2 H2O. It’s important to measure accurately and control conditions well.
Proper waste management is key. HCOOCH CH2 H2O is biodegradable, but bad disposal can harm the air. Follow rules for waste treatment, like incineration or special facilities5. By following these steps, chemists can work safely with HCOOCH CH2 H2O.
Troubleshooting Common Synthesis Problems
Making HCOOCH CH2 H2O can be hard. Chemists often run into problems during the esterification reaction. Let’s look at common issues and how to fix them to make your synthesis better.
Common Issues and Solutions
Low yields are a big problem in making HCOOCH CH2 H2O. This can happen if reactions don’t finish or if unwanted products form. To get better yields, try making the reaction last longer or change how much catalyst you use. Also, getting rid of impurities helps a lot. Clean your starting materials and use clean glassware to avoid contamination.
|
Issue |
Solution |
|---|---|
|
Low yields |
Increase reaction time, adjust catalyst |
|
Impurities |
Purify starting materials, use clean equipment |
|
Side reactions |
Control temperature, optimize reactant ratios |
Quality Control Methods
Keeping your product pure is very important in making HCOOCH CH2 H2O. Use strict testing to keep quality high. Gas chromatography can find impurities. Spectroscopy checks if the product is right. Regular checks help keep your work consistent in labs and factories.
By fixing these common problems and doing good quality checks, you can make your HCOOCH CH2 H2O synthesis better. Remember, practice and careful watching are important to get good at this esterification reaction.
Conclusion: Mastering HCOOCH CH2 H2O Chemistry
HCOOCH CH2 H2O is very important in organic chemistry. It has special properties that are useful in many ways. It dissolves well in water and other liquids, which is great for certain reactions6.
It’s also good for industrial uses because it boils at about 100°C. This means it’s not too volatile but still stable6.
The way HCOOCH CH2 H2O breaks down in water is important. It turns into formic acid and methanol, which is useful in making medicines6. It also helps clean polluted water by removing 85% of harmful stuff6.
HCOOCH CH2 H2O helps the environment too. It makes pesticides safer for the soil and keeps more nutrients in the ground. This helps crops grow better, by up to 20%6.
It’s also good for making cleaner fuels. Adding it to diesel makes engines run 15% better6.
Studying HCOOCH CH2 H2O is very important. It shows how organic chemistry is always changing. By learning about it, scientists can find new ways to make things and help the planet.
FAQ
What is the chemical formula of HCOOCH CH2 H2O?
HCOOCH CH2 H2O is a mix of methyl formate (HCOOCH3) and water (H2O). It’s not one single thing but two different molecules together.
What are the functional groups present in HCOOCH CH2 H2O?
This mix has a carboxyl group (COOH) from methanoic acid. It also has an ester group (COOCH3) from methyl formate. Plus, a hydroxyl group (OH) from water.
How does hydrogen bonding affect the properties of HCOOCH CH2 H2O?
Hydrogen bonding changes this mix a lot. Water helps form bonds with methyl formate’s groups. This affects how it dissolves, boils, and interacts with other molecules.
What is the role of HCOOCH CH2 H2O in esterification reactions?
In esterification, HCOOCH CH2 H2O is key. The methyl formate part is an ester made from methanoic acid and methanol. Knowing this helps us understand ester and hydrolysis reactions in chemistry.
How is HCOOCH CH2 H2O synthesized?
Making this mix starts with mixing methanoic acid and methanol to make methyl formate. Then, water is added. This needs catalysts and careful conditions to work well.
What safety precautions should be taken when handling HCOOCH CH2 H2O?
Handling this mix needs safety gear like gloves and goggles. Good air flow is key because methyl formate is volatile. Always check the Safety Data Sheet (SDS) for safety tips.
What are some industrial applications of HCOOCH CH2 H2O?
Its parts have many uses. Methyl formate is a solvent and fumigant, and helps make formamide. Water’s presence is useful in some processes but might need to be separated in others.
How can the purity of synthesized HCOOCH CH2 H2O be ensured?
Ensuring purity uses methods like gas chromatography (GC) and high-performance liquid chromatography (HPLC). Spectroscopic methods like NMR spectroscopy also help. Regular checks are vital for quality in labs and factories.
Source Links
- Pernithia Galnith: An In-Depth Exploration – https://uprytr.com/pernithia-galnith/
- Bitcoin Attorney Mount Dora Florida: Guide to Legal Representation – https://uprytr.com/bitcoin-attorney-mount-dora-florida/
- iTraderCoin.com: A Revolutionary Approach to Digital Trading – https://theflexshow.com/itradercoin-com/
- Hcooch ch2 h2o: Everything You Need to Know – M5StarsStocks – https://www.m5starsstocks.com/hcooch-ch2-h2o/
- HCOOCH CH2 H2O: Structure, Properties, and Applications – https://teltlk.pro/hcooch-ch2-h2o/
- HCOOCH CH₂ H₂O: The Multifaceted Compound – https://pernithiagalnith.com/hcooch-ch₂-h₂o-the-multifaceted-compound/
- The Star Magazine: Celebrity News, Bios, & Exclusive Interviews – https://www.thestarmagazine.com/
- Cyber Sectors – https://cybersectors.com/
- Top Now – https://topnow.edu.vn/propyl-fomat-a102223
- HCOOCH CH2 H2O: Everything You Need To Know About This Fascinating Compound – The latest tech destination – https://whyntech.co.uk/hcooch-ch2-h2o-everything-you-need-to-know-about-this-fascinating-compound/
- Hcooch Ch2 H2o The Ultimate Guide to Understanding – bbcbusiness.com – https://bbcbusiness.com/hcooch-ch2-h2o-the-ultimate-guide-to-understanding/